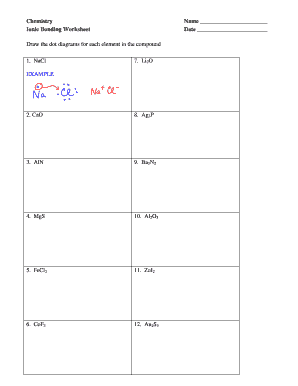
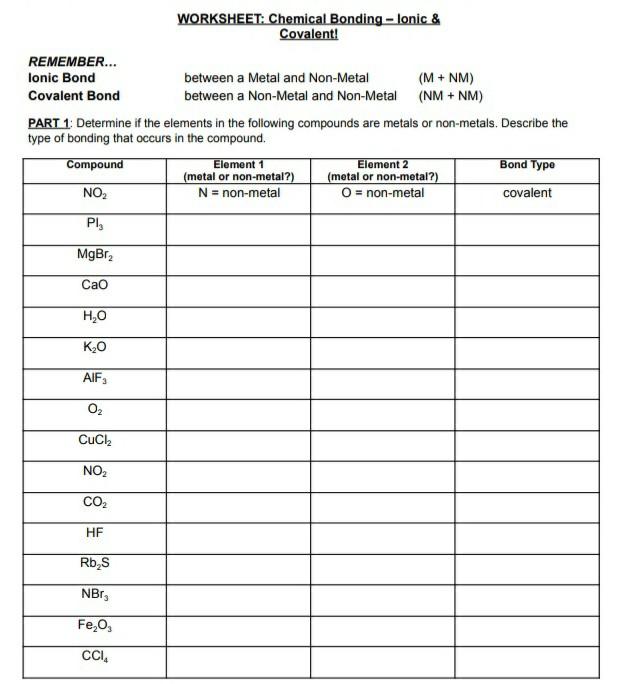
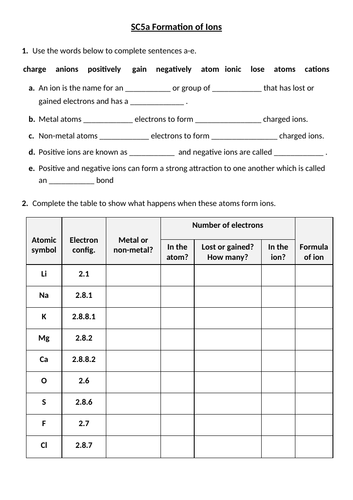
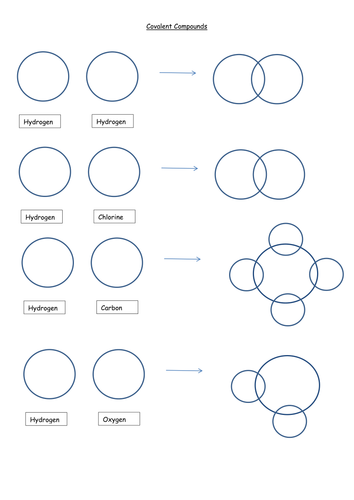
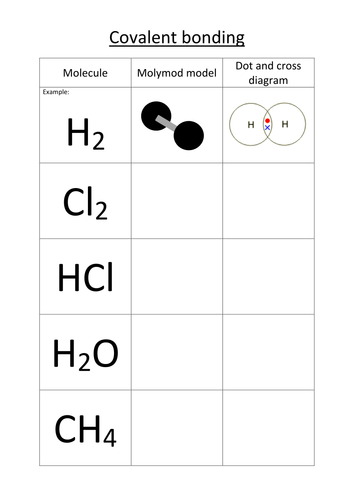
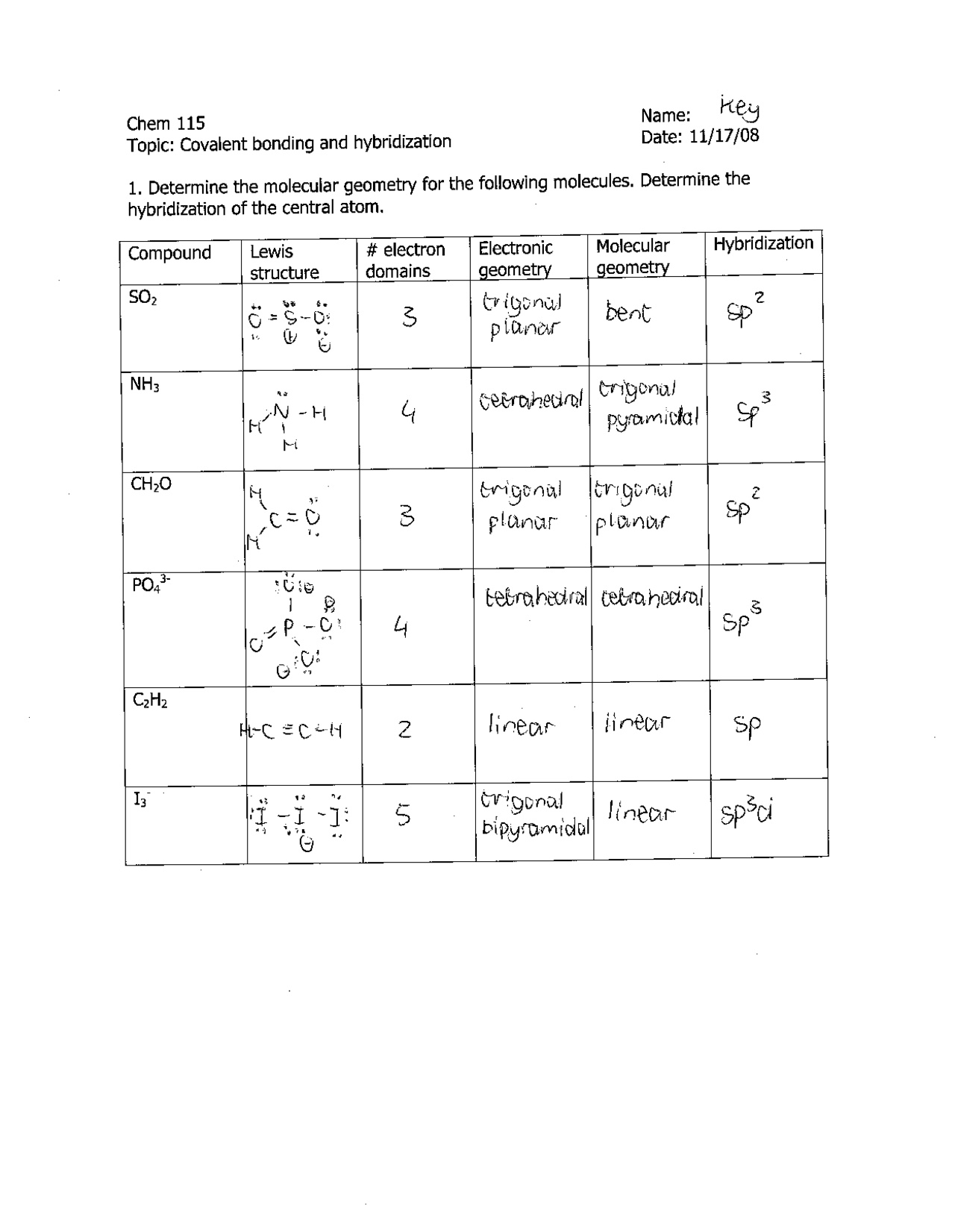
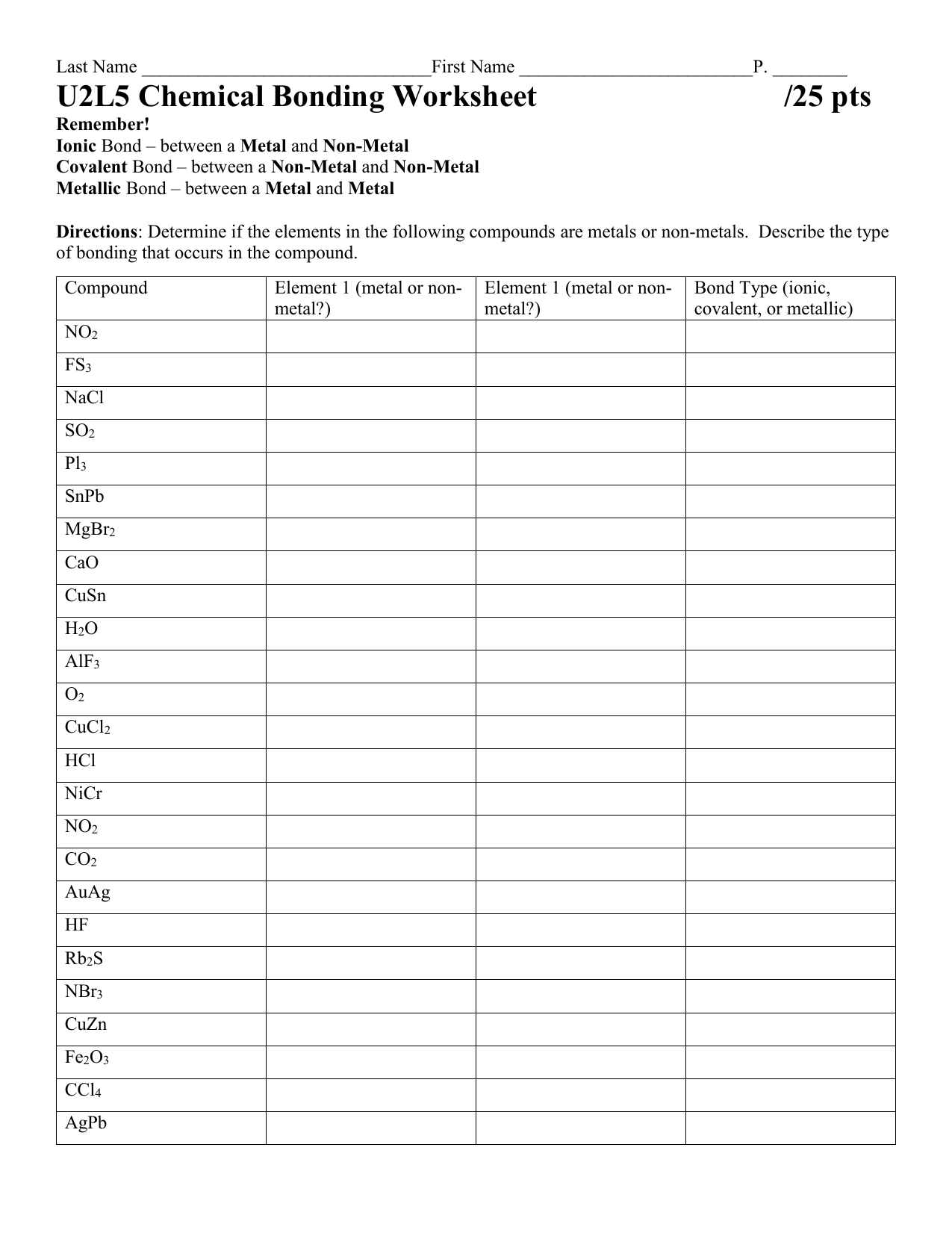
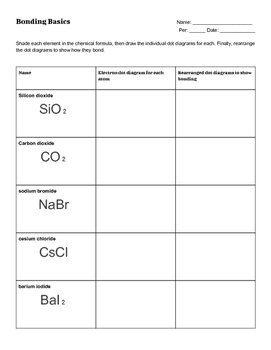
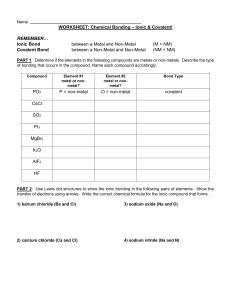
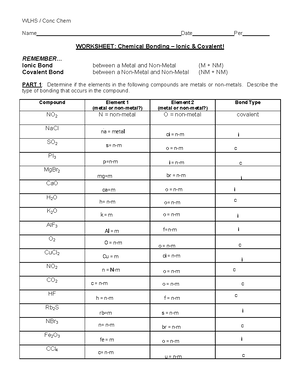
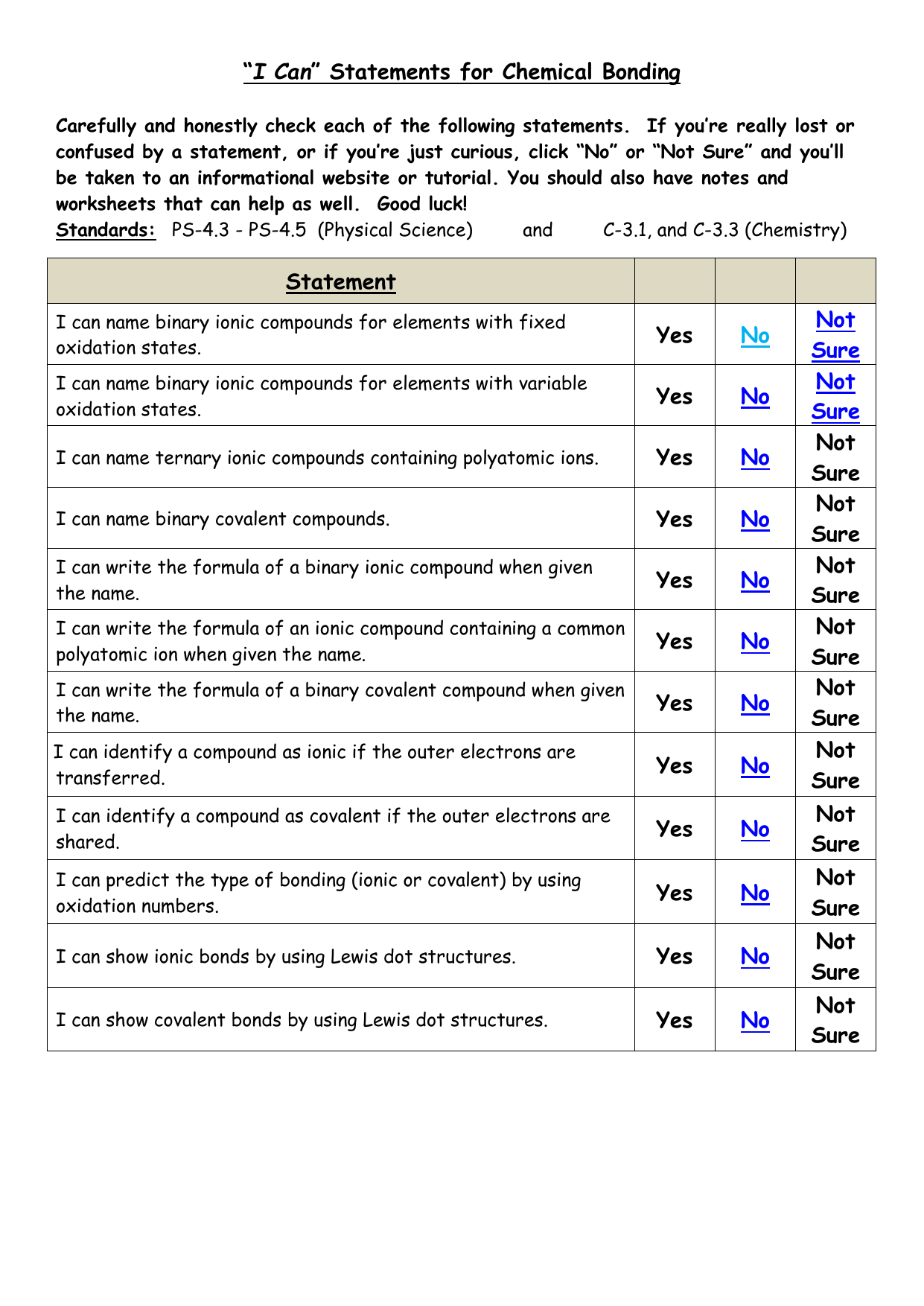
44 worksheet chemical bonding ionic and covalent
› Calculate-Electronegativity3 Ways to Calculate Electronegativity - wikiHow Sep 15, 2022 · If the difference is between 0.5 and 1.6, the bond is polar covalent. These bonds are imbalanced and make the resulting molecule more reactive than one with a nonpolar covalent bond. If the difference is greater than 2.0, the bond is ionic, which means that one atom has a positive charge and the other has a negative one. wordpress.clarku.edu › mat16-caphillips › learningLearning Activity: Ionic or Covalent Bond Experiment LAP – Ionic or Covalent Lab, Day Four LAP – Ionic or Covalent Lab, Day Five. Experiment Handouts: Properties – Ionic and Covalent Compounds Overview: Ionic or Covalent Compound Lab Covalent and Ionic Compounds Lab: PowerPoint. Lab Report Resources: Lab Report Writing Workshop: Powerpoint Peer Review: Feedback Forms. Model Lab Report Lab ...
en.wikipedia.org › wiki › ChemistryChemistry - Wikipedia Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances.

Worksheet chemical bonding ionic and covalent
en.wikipedia.org › wiki › Valence_electronValence electron - Wikipedia To form an ionic bond, a halogen atom can remove an electron from another atom in order to form an anion (e.g., F −, Cl −, etc.). To form a covalent bond, one electron from the halogen and one electron from another atom form a shared pair (e.g., in the molecule H–F, the line represents a shared pair of valence electrons, one from H and ... › difference › Covalent_Bonds_vs_IonicCovalent Bonds vs Ionic Bonds - Difference and Comparison ... Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. Ionic bond, also known as electrovalent bond, is a type of bond formed from the electrostatic attraction between oppositely charged ions in a chemical compound. This ... openstax.org › books › chemistry-2e4.1 Writing and Balancing Chemical Equations - OpenStax Extending this symbolism to represent both the identities and the relative quantities of substances undergoing a chemical (or physical) change involves writing and balancing a chemical equation. Consider as an example the reaction between one methane molecule (CH 4 ) and two diatomic oxygen molecules (O 2 ) to produce one carbon dioxide ...
Worksheet chemical bonding ionic and covalent. edu.rsc.org › resourcesTeaching resources | RSC Education Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities openstax.org › books › chemistry-2e4.1 Writing and Balancing Chemical Equations - OpenStax Extending this symbolism to represent both the identities and the relative quantities of substances undergoing a chemical (or physical) change involves writing and balancing a chemical equation. Consider as an example the reaction between one methane molecule (CH 4 ) and two diatomic oxygen molecules (O 2 ) to produce one carbon dioxide ... › difference › Covalent_Bonds_vs_IonicCovalent Bonds vs Ionic Bonds - Difference and Comparison ... Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. Ionic bond, also known as electrovalent bond, is a type of bond formed from the electrostatic attraction between oppositely charged ions in a chemical compound. This ... en.wikipedia.org › wiki › Valence_electronValence electron - Wikipedia To form an ionic bond, a halogen atom can remove an electron from another atom in order to form an anion (e.g., F −, Cl −, etc.). To form a covalent bond, one electron from the halogen and one electron from another atom form a shared pair (e.g., in the molecule H–F, the line represents a shared pair of valence electrons, one from H and ...



























0 Response to "44 worksheet chemical bonding ionic and covalent"
Post a Comment