38 moles and mass worksheet
› Mole › Determine-formula-ofDetermine the formula of a hydrate: fifteen examples - ChemTeam Two moles of HCl react for every one mole of carbonate. Therefore: 0.0224 mole / 2 = 0.0112 mol of carbonate. 4) Determine the mass of 0.0112 mol of Na 2 CO 3. (0.0112 mol Na 2 CO 3) (105.988 g Na 2 CO 3 / 1 mol) = 1.187 g Na 2 CO 3. This is how many grams of anhydrous sodium carbonate dissolved. 5) Mass of hydrated salt − mass of anhydrous ... › Solutions › Molality-probs1-toChemTeam: Molality Problems #1-15 Problem #9: Calculate the molality (m) of a 7.55 kg sample of a solution of the solute CH 2 Cl 2 (molar mass = 84.93 g/mol) dissolved in the solvent acetone (CH 3 COH 3 C) if the sample contains 929 g of methylene chloride Solution: mass solvent ---> 7550 g − 929 g = 6621 g = 6.621 kg moles solute ---> 929 g/ 84.93 g/mol = 10.9384 mol
assignmentessays.comAssignment Essays - Best Custom Writing Services Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
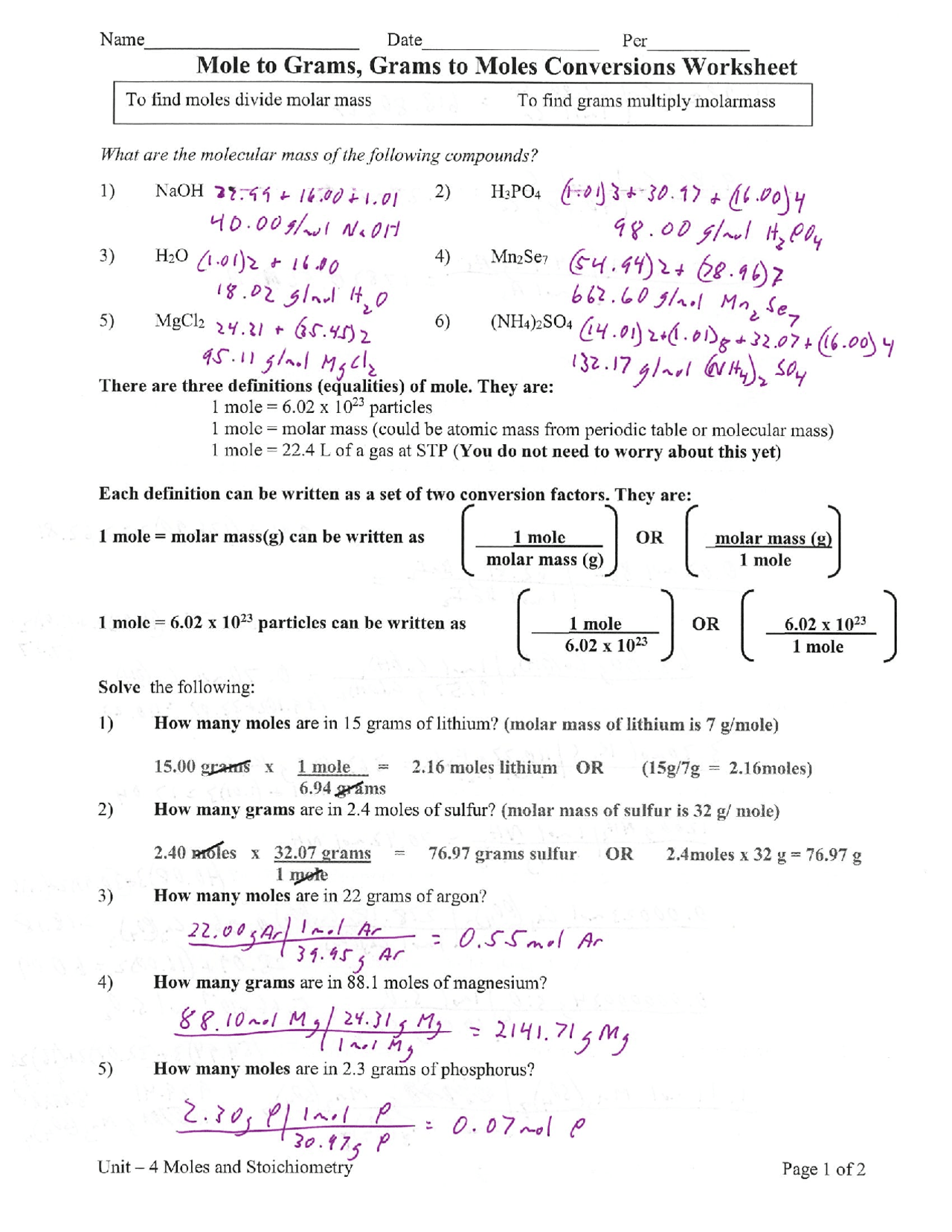
Moles and mass worksheet
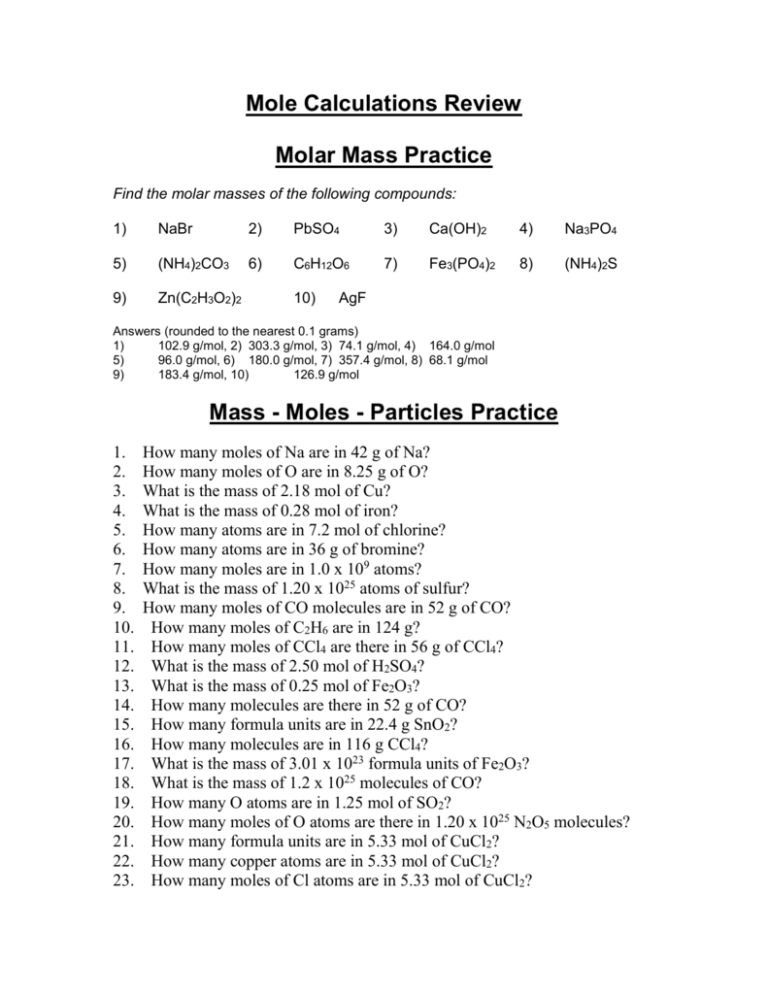
› article › academics-the-artsHow to Calculate the Empirical Formula of a Compound - dummies Jul 21, 2021 · Convert the masses from Step 1 into moles using the molar mass. Determine which element has the smallest mole value. Then divide all the mole values you calculated in Step 2 by this smallest value. This division yields the mole ratios of the elements of the compound. › printable-chemistry-worksheetsFree PDF Chemistry Worksheets To Download or Print - ThoughtCo May 30, 2019 · Temperature Conversions Worksheet; Temperature Conversions Answers; Temperature Conversions Worksheet #2; Temperature Conversions Answers #2; Moles to Grams Conversions Worksheet; Moles to Grams Conversions Answers; Formula or Molar Mass Worksheet; Formula or Molar Mass Worksheet Answers; Practicing Balancing Chemical Equations - Worksheet › Solutions › Molarity-probs1-10ChemTeam: Molarity Problems #1 - 10 MV = grams / molar mass (x) (1.000 L) = 245.0 g / 98.0768 g mol¯ 1. x = 2.49804235 M to four sig figs, 2.498 M If the volume had been specified as 1.00 L (as it often is in problems like this), the answer would have been 2.50 M, NOT 2.5 M. You want three sig figs in the answer and 2.5 is only two SF.
Moles and mass worksheet. › molecular-mass-calculationsMolecular Mass Calculations - ThoughtCo Mar 11, 2019 · Find the atomic mass for each element by using the mass given in the Periodic Table. Multiply the subscript (number of atoms) times the atomic mass of that element and add the masses of all of the elements in the molecule to get the molecular mass. For example, multiple the subscript 12 times the atomic mass of carbon (C). › Solutions › Molarity-probs1-10ChemTeam: Molarity Problems #1 - 10 MV = grams / molar mass (x) (1.000 L) = 245.0 g / 98.0768 g mol¯ 1. x = 2.49804235 M to four sig figs, 2.498 M If the volume had been specified as 1.00 L (as it often is in problems like this), the answer would have been 2.50 M, NOT 2.5 M. You want three sig figs in the answer and 2.5 is only two SF. › printable-chemistry-worksheetsFree PDF Chemistry Worksheets To Download or Print - ThoughtCo May 30, 2019 · Temperature Conversions Worksheet; Temperature Conversions Answers; Temperature Conversions Worksheet #2; Temperature Conversions Answers #2; Moles to Grams Conversions Worksheet; Moles to Grams Conversions Answers; Formula or Molar Mass Worksheet; Formula or Molar Mass Worksheet Answers; Practicing Balancing Chemical Equations - Worksheet › article › academics-the-artsHow to Calculate the Empirical Formula of a Compound - dummies Jul 21, 2021 · Convert the masses from Step 1 into moles using the molar mass. Determine which element has the smallest mole value. Then divide all the mole values you calculated in Step 2 by this smallest value. This division yields the mole ratios of the elements of the compound.




























0 Response to "38 moles and mass worksheet"
Post a Comment