40 13.1 the nature of gases worksheet
PDF What do I already know about states of matter? (index card) and ... The Nature of Gases > Kinetic Theory and a Model for Gases According to kinetic theory: •The particles in a gas are considered to be small, hard spheres with an insignificant volume. •The motion of the particles in a gas is rapid, constant, and random. •All collisions between particles in a gas are perfectly elastic. 13.1 Read Free Chapter 13 States Of Matter Practice Problems Answers sure Torricelli Section 13.1 The Nature of Gases The SI unit of pressure is the pas- cal (Pa) At sea level, atmospheric pres- sure is about 101.3 kilopascals (kPa) Old- er units of pressure include millimeters of mercury (mm Hg),... Chapter 13 States of Matter - Chapter 13 States of Matter ... Chemistry Chapter 13: States Of Matter Review.
PDF 13.1 Wildfires And Prescribed Burning - US EPA 10 µm, probably of ash and partially burned plant matter, are entrained by the turbulent nature of high-intensity fires. Burning methods differ with fire objectives and with fuel and weather conditions.4 For example, the various ignition techniques used to burn under standing trees include: (1) heading fire, a

13.1 the nature of gases worksheet
An Introduction to Chemistry - Atoms First 1st edition 11.2: Ideal Gas Calculations (34) 11.3: Equation Stoichiometry and Ideal Gases (16) 11.4: Dalton's Law of Partial Presssures (9) Advanced Problem ; Advanced Problem (7) Chapter 12: Liquids: Condensation, Evaporation, and Dynamic Equilibrium 12.1: Changing from Gas to Liquid and from Liquid to Gas - An Introduction to Dynamic Equilibrium (18) Chapter 13 ~ Non-Renewable Resources - Environmental Science Canada is one of the world's leading producers of metals, accounting for 15% of the global production of nickel in 2006, 9% of aluminum, and 6% zinc (Tables 13.1 and 13.2). Much metal production is intended for export. Domestic consumption is about 39% of the value of production of all metals (Table 13.2). Glencoe Physical Science 2012 Student Edition (Glencoe ... - bartleby Textbook solutions for Glencoe Physical Science 2012 Student Edition (Glencoe… 1st Edition Charles William McLaughlin and others in this series. View step-by-step homework solutions for your homework. Ask our subject experts for help answering any of your homework questions!
13.1 the nature of gases worksheet. 13: Properties of Solutions - Chemistry LibreTexts 13.1: The Solution Process Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic scale. The component present in the greatest amount is the solvent, and the components present in lesser amounts are the solute (s). PDF Chemistry: Matter and Change 13.1 The Gas Laws The Combined Gas Law (cont.) SECTION 13.1 The Gas Laws Boyle's Law explains which relationship of properties in gases? A. pressure and volume B. amount and pressure C. temperature and volume D. volume and temperature SECTION 13.1 Section Check Atoms are in their lowest energy state at what temperature? A. 0° Celsius 13.1 Acids and bases | Types of reactions | Siyavula Using the common acids and bases in Table 13.1 , pick an acid and a base from the list. Write a chemical equation for the reaction of these two compounds. Now identify the conjugate acid-base pairs in your chosen reaction. Compare your results to those of your classmates. Video: 245N. Site To Download Chapter 13 Electrons In Atoms Worksheet Answers Atom Section Review 13.1 1. List in chronological order, a major contribution of each of these scientists to the understanding of the atom: proposed that all elements are composed of atoms.Dal- ton - Chapter 13 Electrons in Atoms - Mrs. Morales PEP site 12. The maximum # of electrons allowed in an energy level =
PDF Chapter 13- The States of Matter 13.1- The Nature of Gases In a closed container the gas molecules will cause pressure. The pressure at equilibrium is called vapor pressure Different compounds have different vapor ... 13.3- The Nature of Liquids 13.4 Phase Digrams Solids- definite volume and shape, high density Solids and Liquids have high densities because their molecules are close together. Honors Chemistry - PLHS Science 12.1 Chemical Calculations 12.3 Limiting Reagent & Percent Yield . Chapter 13 - States of Matter 13.1 The Nature of Gases 13.2 The nature of Liquids 13.3 The Nature of Solids 13.4 Changes of State Changes of State Lab Chapter 14 - Gases 14.1 Properties of Gases 14.2 The Gas Laws 14.3 Ideal Gases 14.4 Gases: Mixtures and Movements Chemistry (12th Edition) Chapter 13 - States of Matter - 13.1 The ... Chapter 13 - States of Matter - 13.1 The Nature of Gases - 13.1 Lesson Check - Page 424: 7 Answer a)96.235 kPa b)5.998 kPa Work Step by Step Please check attached image for step by step explanation of how the answers were obtained. Update this answer! You can help us out by revising, improving and updating this answer. Update this answer 13.1 Nature of Gases Worksheet.pdf - Name Date Class THE... View 13.1 Nature of Gases Worksheet.pdf from CHEMISTRY 163 at Rowad AlKhaleej International Schools, Dammam. Name Date Class THE NATURE OF GASES Section Review Objectives Describe the assumptions of
PDF CK-12 Chemistry Contents Contents 1 Introduction to Chemistry Assessments1 1.1 What is Chemistry ... Gases and Pressure Worksheet.pdf - Gases and Pressure Name... 1. Mercury is the liquid used in a barometer over water because mercury is 13.6 times moredense than water. This means that an equal mass of water requires 13.6 times as much spaceso its height in the tube would be 13.6 times greater than the height of mercury. PDF Chapter 13 - Gases Exercise 13.1 - Using the Ideal Gas Equation: Krypton gas does a better job than argon of slowing the evaporation of the tungsten filament in an incandescent light bulb. Because of its higher cost, however, krypton is used only when longer life is considered to be worth the extra expense. (Objs 15, 16, & 17) a. Pearson Chemistry - 9780132525763 - Solutions and Answers - Quizlet The Nature of Covalent Bonds. Section 8.3: Bonding Theories. Section 8.4: Polar Bonds and Molecules . Page 256: Assessment. Page 261: Standardized Test Prep. Exercise 1. ... The Nature of Gases. Section 13.2: The Nature of Liquids. Section 13.3: The Nature of Solids. Section 13.4: Changes of State. Page 443: Assessment. Page 447: Standardized ...
13.S: Properties of Solutions (Summary) - Chemistry LibreTexts 13.1.1 Energy Changes and Solution Formation. overall enthalpy change in formation of a solution. Δ H s o l n = Δ H 1 + Δ H 2 + Δ H 3. Δ H 1 = separation of solute molecules. Δ H 1 = separation of solvent molecules. Δ H 3 = formation of solute-solvent interactions. separation of solute particles is endothermic.
Online Library Nature Of Liquids Section Review Key SECTION 13.1 THE NATURE OF GASES 1.. To answer the following questions,. Pren- tice Hall Chemistry Worksheets Author: Solids Liquids and Gases ANSWER KEY DPS109. Worksheet 1 Answers will vary. understand the particle nature of matter and. Gases States of Matter Enrich 1.. Tri- cia's Compilation for 'chapter 13 the na-
Chemistry (12th Edition) Chapter 13 - States of Matter - GradeSaver Answer 1)All particles in the liquid should have the kinetic energy that allows them to break the intermolecular forces of attraction and escape. 2)vapor pressure of liquid should be equal or more than the external pressure on the surface of the liquid. Work Step by Step Refer to page 428 the answer to the key question. Update this answer!
CK-12 Chemistry Contents Contents 1 Introduction to Chemistry Worksheets1 1.1 What is Chemistry ...
Chapter 13 gases are similar, and one way in which they are different. Both liquids and gases can flow, so they can take the shape of their container. The molecules in a liquid have intermolecular attractions that are not present in gases. Therefore, liquids have a definite volume and will not simply fill their container.
PDF Name Date Class STATES OF MATTER 13 SECTION 13.1 THE NATURE OF GASES (pages 385-389) This section introduces the kinetic theory and describes how it applies to gases. It defines gas pressure and explains how temperature is related to the kinetic energy of the particles of a substance. Kinetic Theory and a Model for Gases (pages 385-386) 1.
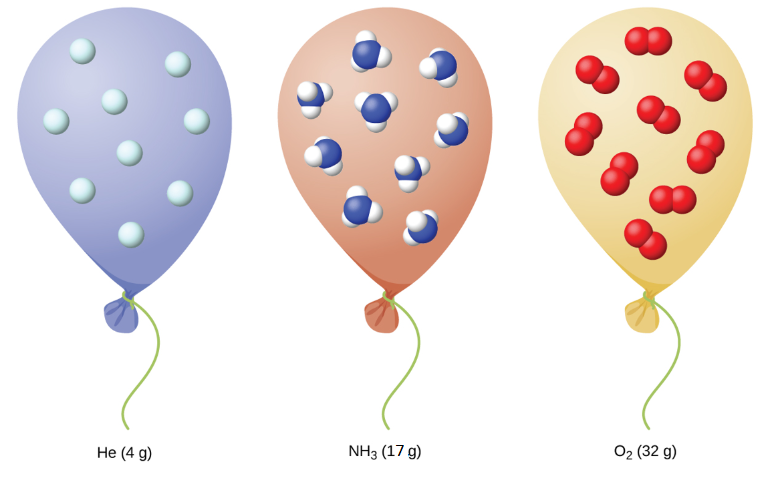
PDF Chapter 13 Gases - An Introduction to Chemistry Gases consist of tiny particles widely spaced (Figure 13.1). Under typical conditions, the average distance between gas particles is about ten times their diameter. Because of these large distances, the volume occupied by the particles themselves is very small compared to the volume of the empty space around them.
Answer Keys - HONORS CHEMISTRY Hess's Law Worksheet SG 16.4 SG 16.3 & 16.5 Gibbs Free Energy Chapter 16 Review Reviewing Vocabulary Chapter 19 Section 19.1 Review SG 19.1 & 19.2 Understanding Logarithms SG 19.3 Determining pH & pOH SG 19.4 Neutralization Reactions Titrations Practice Worksheet Acid Base Lab Chapter 19 Review Worksheet Titration Lab Quiz 19.4 ...
The Nature of Gases 13.1 Flashcards | Quizlet The Nature of Gases 13.1. the kinetic theory describes the what? Click card to see definition 👆. motion of particles in matter and the forces of attraction between them. Click again to see term 👆. 1/27. Previous. ←. Next.
PDF Chem ID# Name CHAPTER 13 STATE OF MATTER SECTION 13.1 THE NATURE OF GASES CHAPTER 14 GAS LAWS 1) A bag of potato chips is packaged at sea level (1.00 atm) and has a volume of 0.315 L. If this bag of chips is transported to Denver (0.775 atm), what will the new volume of the bag be? 2) A Los Angeles class nuclear submarine has an internal volume of eleven million liters at a pressure of 126.62 kPa. If a crewman were to open one of the hatches to the outside ocean ...
Glencoe Physical Science 2012 Student Edition (Glencoe ... - bartleby Textbook solutions for Glencoe Physical Science 2012 Student Edition (Glencoe… 1st Edition Charles William McLaughlin and others in this series. View step-by-step homework solutions for your homework. Ask our subject experts for help answering any of your homework questions!
Chapter 13 ~ Non-Renewable Resources - Environmental Science Canada is one of the world's leading producers of metals, accounting for 15% of the global production of nickel in 2006, 9% of aluminum, and 6% zinc (Tables 13.1 and 13.2). Much metal production is intended for export. Domestic consumption is about 39% of the value of production of all metals (Table 13.2).
An Introduction to Chemistry - Atoms First 1st edition 11.2: Ideal Gas Calculations (34) 11.3: Equation Stoichiometry and Ideal Gases (16) 11.4: Dalton's Law of Partial Presssures (9) Advanced Problem ; Advanced Problem (7) Chapter 12: Liquids: Condensation, Evaporation, and Dynamic Equilibrium 12.1: Changing from Gas to Liquid and from Liquid to Gas - An Introduction to Dynamic Equilibrium (18)





























0 Response to "40 13.1 the nature of gases worksheet"
Post a Comment