45 reaction products worksheet answers
DOC Answers for Predicting Products of Chemical Reactions In this question, you must recognize that perchlorate, ClO4-, and hydroxide, OH-, are polyatomic ions and will not break apart. Also, this is an acid-base reaction, so the products should be salt and water. 4. Decomposition: ZnCO3 + heat ( ZnO + CO2. When reactions have heat as a reactant, it is very likely that they will involve decompositions. Reaction Products Worksheets - Answer Key (2).doc a voyage through equations answer key section 1: identify the type of reaction 1) na3po4+ 3 koh 3 naoh + k3po4 double displacement 2) mgcl2+ li2co3 mgco3+ 2 licl double displacement 3) c6h12+ 9 o2 6 co2+ 6 h2o combustion 4) pb + feso4 pbso4+ fe single displacement 5) caco3 cao + co2 decomposition 6) p4+ 3 o2 2 p2o3 synthesis 7) 2 rbno3+ bef2 be …
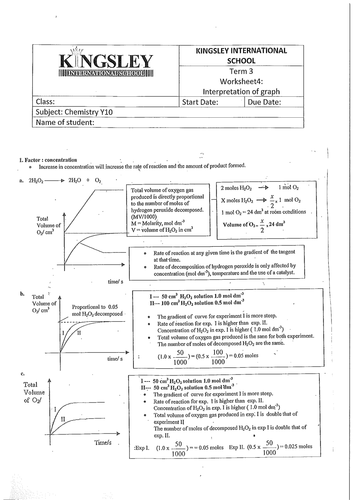
› _cache › filesKINETICS Practice Problems and Solutions a. The concentration of the catalyst will go down as the reaction proceeds. b. The catalyst provides a new pathway in the reaction mechanism. c. The catalyst speeds up the reaction. d. Two of the above. e. None of the above. 7. The catalyzed reaction has a _____ activation energy and thus causes a _____ reaction rate. a. higher, lower b. higher ...
Reaction products worksheet answers
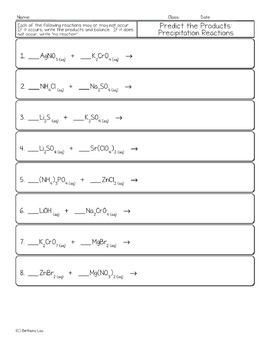
Predicting_Reaction_Products_Worksheet - Predicting... Predicting Reaction Products Predict the products for the following reactions, balance the equation, then classify the type of reaction: 1) ____ Na + ____ FeBr 3 ? 2) ____ NaOH + ____ H 2 SO 4 ? 3) ____ C 2 H 4 O 2 + ____ O 2 ? 4) ____ NH 3 + ____ H 2 O 5) ____ PbSO 4 + ____ AgNO 3 ? 6) ____ PBr 3 ? PDF Answers for Predicting Products of Chemical Reactions This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and combustion reactions. For the first few reactions, the type of reaction is listed, you should predict the products, then balance. Further questions just have Reactions Double Worksheet Answers Replacement Single And Watch all of the following videos Single Replacement Reaction Worksheet Answers Each half-reaction starts with the same reactant Predicting Products of Chemical Reactions - This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double ...
Reaction products worksheet answers. Predicting Reaction Products Worksheet Key - Predicting Reaction ... Predicting Reaction Products - Solutions. Balance the equations and predict the products for the following reactions: 3 Na + 1 FeBr 3 3 NaBr + 1 Fe; 2 NaOH + 1 H 2 SO 4 1 Na 2 SO 4 + 2 H 2 O; 1 C 2 H 4 O 2 + 2 O 2 2 CO 2 + 2 H 2 O; 1 NH 3 + 1 H 2 O 1 NH 4 OH; 1 PbSO 4 + 2 AgNO 3 1 Ag 2 SO 4 + 1 Pb(NO 3 ) 2; 4 PBr 3 1 P 4 + 6 Br 2 141 Predicting Products of Reactions Key - StuDocu Predicting Products of Reactions Key. PART A: Predict the Products (includes identifying type of reaction) and Balance: 2 C 8 H 18 (l) + 25 O 2 (g) 16 CO 2 (g) + 18 H 2 O (g) Type of Reaction: combustion usscouts.org › mb › worksheetsChemistry - USSCOUTS.ORG c c. Visit an industrial plant that makes chemical products or uses chemical processes and describe the processes used. What, if any, by-products are produced and how they are handled. c d. Visit a county farm agency or similar governmental agency and learn how chemistry is used to meet the needs of agriculture in your county. Chemical Reactions Worksheets - 12 sets of [MCQ] & other - all solved 1. substance produced in a chemical reaction. 2. force of attraction that breaks and reforms in a chemical reaction. 3. substance that starts a chemical reaction. 4. example of chemical change. 5. balance between opposing changes. 6. process in which some substances become different substances. 7. example of a physical change.
gosciencegirls.com › balancing-equations100 Balancing Chemical Equations Worksheets with Answers ... Oct 02, 2019 · This signifies a reaction which is irreversible or is unchangeable after a certain stage. However, in certain situations, the reactions occur at equilibrium. This means that reaction at any forward rate results in a reverse reaction. In such situations, the arrow used is two-sided, i.e. facing towards the reactants and the products. bluu.myvoicehosting.itNuclear reaction practice worksheet answers - If values 5: Chemical Equations. If values of three variables are known, then the others can be calculated using the#[email protected] abc gb ofo dnq db de ecd psn ae gnbn aljn eh lj ag eba abbb aa abb kkbm dk lpf aa meo gc dba jt eej ccdf aaaa fb mom Reactions Double Worksheet Answers Replacement Single And Watch all of the following videos Single Replacement Reaction Worksheet Answers Each half-reaction starts with the same reactant Predicting Products of Chemical Reactions - This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double ... PDF Answers for Predicting Products of Chemical Reactions This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and combustion reactions. For the first few reactions, the type of reaction is listed, you should predict the products, then balance. Further questions just have
Predicting_Reaction_Products_Worksheet - Predicting... Predicting Reaction Products Predict the products for the following reactions, balance the equation, then classify the type of reaction: 1) ____ Na + ____ FeBr 3 ? 2) ____ NaOH + ____ H 2 SO 4 ? 3) ____ C 2 H 4 O 2 + ____ O 2 ? 4) ____ NH 3 + ____ H 2 O 5) ____ PbSO 4 + ____ AgNO 3 ? 6) ____ PBr 3 ?











0 Response to "45 reaction products worksheet answers"
Post a Comment