39 emission spectra and energy levels worksheet
Experiment 1: Speed of molecules Part A: Add 50 heavy gas ... Sound waves and light waves carry energy that enable youThe purpose of this lab was to determine what the speed of the sound of air was in the classroom. The virtual ports in ESX Server provide a rich control channel for The Virtual Physical Laboratory is a suite of 370 interactive physics simulations. Procedure: 1. 10 IT Essentials 7. Sep 15, 2020 · They use … Absorption and Emission Spectra - PhysicsLAB Absorption and Emission Spectra. Directions: On this worksheet you will practice your knowledge of the properties of absorption and emission spectra as well as several historical experiments leading to their understanding. omit. Question 1 An experiment is performed on a sample of atoms that have a ground state energy of -38 eV.
Lesson Worksheet:Emission and Absorption Spectra | Nagwa In this worksheet, we will practice determining the composition of a material from the features that appear in the spectrum of light coming from it. Q1: A scientist has a sample of an unknown gas. In order to identify it, she shines a continuous spectrum of white light through the gas and observes which wavelengths of light are absorbed by it.

Emission spectra and energy levels worksheet
PDF Flame Test Lab Activity Key - University of South Florida wavelength of 459 nm. What is the energy content, in joules, of this photon? (-34 8)( )-19-7-19 6.33 x 10 J s 3.00 x 10 m s = = 4.33 x 10 J 4.59 x 10 m = 4.33 x 10 J E E ⋅ 3. Emission spectrums are produced from the release of light by electrons in varying energy levels. The following is an emission spectrum of hydrogen gas. Calculate the Lesson Worksheet:Electron Energy Levels | Nagwa Lesson Worksheet: Electron Energy Levels. In this worksheet, we will practice determining whether an electron shell of an atom is filled and which electron transitions are possible in a given atom. The diagram shows a hydrogen atom. The electron shown transitions between two energy levels of the atom. study.com › learn › bohr-model-questions-and-answersBohr Model Questions and Answers - Study.com In the spectrum of atomic hydrogen, a violet line from the Balmer series is observed at 434 nm. Determine the beginning and ending energy levels of the electron during the emission of energy that l...
Emission spectra and energy levels worksheet. rvpconsultant.us › unit-2-worksheet-3-physics-answersrvpconsultant.us Oct 28, 2014 · Unit 2 worksheet 3 physics answers. email protected] [email protected] Quiz & Worksheet - Line Emission Spectra | Study.com Worksheet Print Worksheet 1. Which of the following best describes the reason that line emission spectra contain lines? The energy levels in an atom are discrete and only certain emission... Energy Levels and Spectra - Physics Things Calculate the energy difference (in Joules) between energy levels. Using either E = hf, E = hc/wavelength or for electrons E = 1/2mv^2 you can work out the energy of the incident photon/electron. If this energy is equal to the difference in energy levels, the electron will be excited. Solution for Student Worksheet: Energy Levels in the Atom Solution for Calculate the Energy! Student Worksheet Neils Bohr numbered the energy levels (n) of hydrogen, with level 1 (n=1) being the ground state, level 2 being the first excited state, and so on.Remember that there is a maximum energy that each electron can have and still be part of its atom. Beyond that energy, the electron is no longer bound to the nucleus of the atom and it is ...
gps-tracker-fuers-fahrrad.de Labflow pre lab answers filmreview.us › unit-2-worksheet-3-physics-answersfilmreview.us Oct 02, 2013 · Each line is one unit. unit worksheet clearly explain what is meant the term ans) refraction is the bending of light as it passes across the boundary between two different Constant Velocity Particle Model Worksheet 2: shown below at left. J of heat are applied to 10. A kilometer is equal to 1,000 meters, and a kilogram is equal to 1,000 grams. Spectra and energy levels | Teaching Resources Spectra and energy levels. Subject: Physics. Age range ... doc, 128 KB doc, 35 KB doc, 22 KB doc, 27 KB doc, 69.5 KB. A series of discussions and demonstrations looking at spectra and emission levels. Tes classic free licence. Review. 5 ... Intro to quanta using line spectra and LEDs. Lots of background info for the teacher, questions (+answers ... PDF Emission Spectra and Energy Levels energy produced by the movement of an electron from one discrete energy level to another. Thus, emission spectra are experimental proof that electrons exist at definite, distinctive energy levels in an atom. Problem: In this experiment, you will study the emission spectra of three elements: hydrogen, neon, and helium. You will
PDF Atomic Emission Spectra Atomic Emission Spectra The electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. The ground state of an atom is the lowest energy state of the atom. When those atoms are given energy, the electrons absorb the energy and move to a higher energy level. filmreview.us › analyzing-atomic-spectra-worksheet-answersfilmreview.us A mass spectrum of Atomic Spectra Worksheet Key Directions In this exercise you will be given some terminology to define. 001. 3 atomic emission spectra worksheet answers, interpret emission spectra worksheet answers, atomic emission spectra worksheet answers, emission spectra and energy levels worksheet answers, worksheet emission and ... Emission Spectra and Energy Levels Worksheet - Studyres Name: _____ Period:_____ Page # _____ Emission Spectra and Energy Levels Worksheet Discusion: One convenient method of exciting atoms of an element is to pass an electric current through a sample of the element in the vapor phase. This is the principle behind the spectrum tubes in the demonstration. Chapter 4 Arrangement of Electrons in Atoms Bright-Line Spectra. Emission Spectra and. Energy Levels. Worksheet. Discussion: One convenient method of exciting atoms of an element is to pass an ...22 pages

Emission Spectra Worksheet | Printable Worksheets and Activities for Teachers, Parents, Tutors ...
Student Worksheet: Graphing Spectra - NASA Student Worksheet: Graphing Spectra Solution for Graphing Spectra Student Worksheet, Part II The graphical representation should include all visible lines shown in the color specrum. The continuum should rise gradually from 4000 angstroms, and remain fairly constant through blue, and decrease slightly in green portion of the spectrum.
2 Emission Spectra Energy Levels and Spectral Charts-1.pdf ... Thus, emission spectra are experimental proof that electrons exist in definite, distinctive energy levels in an atom. Questions: 1. How can the difference in the brightness of spectral lines be explained? 2. According to the modern theory of the atom, where may an atom's electrons be found? 3. How do electrons become "excited"? 4.
PDF Electrons Energy and Light WS - gardencity.k12.ny.us His model included electrons orbiting the nucleus at specific energy levels. Electrons absorb energy from various sources (electricity) when they move from lower energy levels (ground state) to higher energy levels (excited states). Energy is released as electrons return to their lower energy levels. 18.
Solved Atomic Emission Spectra Worksheet Part I: | Chegg.com Atomic Emission Spectra Worksheet Part I: Calculations of Electron Energy Levels (E.) in the Bohr Atom of Hydrogen bebe Each shell in the Bohr atom has a positive integer value "n" that represents an energy level for that orbit.
EmissionSpectraEnergyLevelsPractice.pdf - Name Date Pd ... movement of an electron from one discrete energy level to another. Thus, emission spectra are experimental proof that electrons exist in definite, distinctive energy levels in an atom. Questions: 1. How can the difference in the brightness of spectral lines be explained? The thickness (brightness) depends on the number of photons.
PDF Worksheet 1.3 Emission spectra and electron configurations No. Question Answer 1In which region of the electromagnetic spectrum would you find lines of the greatest energy—ultraviolet, visible or infrared? 2On the energy level diagram show the electron transitions that would result in the series of lines that are seen in the visible region of the hydrogen emission spectrum.
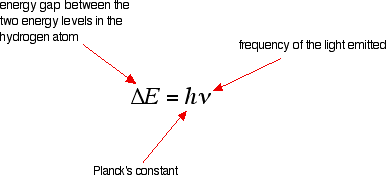
Chemistry 101 8-ATOMIC EMISSION SPECTRA excited absorb ... relationship between the energy and the frequency and wavelength is given by the following equation: E final- E initial= ∆E = hυ = hc/λ (2) The quantity his known as Planck's constant and is equal to 6.626 x 10-34J ⋅ s (J = joule, s = second). The emission spectrum consists of discrete lines corresponding to the differences in
Vibrational energy chart - benandmarina.us email protected]





0 Response to "39 emission spectra and energy levels worksheet"
Post a Comment