42 specific heat worksheet answers
specific.” – They are not used up in reactions, so they can be used again & again – They are substrate-specific (each enzyme’s active site has a specific shape that only fits a certain substrate=substance the enzyme breaks down or assembles Calculating Specific Heat Worksheet Answers Specific Heat Worksheet Name (in ink): C = q/mAT, where q = heat energy, m = mass, and T = temperature Remember, AT = (Tfinal — Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat energy ...
Specific Heat Worksheet Cp = q/mAT, where q = heat energy, m = mass, and T = temperature A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 250C to 1750C. Calculate the heat capacity of iron .cp 5 Cp How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 220C
Specific heat worksheet answers
A 1575 g piece of iron sorbs 108675 joules of heat energy and its temperature changes from 25 0 1750c. The mass of the water is 70 0 g. When the chemical reactions reach a specific temperature they are said to be complete. 100 0 ml of 4 0 c water is heated until its temperature is 37 c. Specific heat chem worksheet 16 1 answer key. Answers are provided at the end of the worksheet without units. O nb 25 a 75 0 g sample of a metal at is heated by adding 450 j of heat. Chemistry worksheet name specific heat capacity block. 4 18 j g 0 c 1 00 j g 0 c 0 80 j g 0 c. Calculate the energy required to heat a beaker of water at 18 c to boiling. This is an utterly simple. Specific Heat and Heat Capacity Worksheet 1 The temperature of 335 g of water changed from 24.5oC to 26.4oC.How much heat did this sample absorb? c for water = 4.18 J/goC (ans. 2.66 kJ) 2.
Specific heat worksheet answers. Specific heat worksheet with answers. Show all work and proper units. Heat is not the same as temperature yet they are related. Calculate the specific heat capacity of iron. Identify each variables by name the units associated with it. Specific heat calculations worksheet answers specific heat worksheet name in ink. Specific Heat Worksheet With Answers Pdf. January 9, 2022. The specific heat of water is 4 18 j g c 3. A block of aluminum weighing 140 g is cooled from 98 4 c to 62 2 c with the release of 1080 joules of heat. Right Triangle Word Problems Worksheet In 2020 Word Problem Worksheets Word Problems Triangle Worksheet. What is the symbol for specificheat? _. S. Look at the specific heat capacity chart on the first page of this worksheet. Which ...4 pages Heat capacities depend upon the mass of the sample, so the specific heat, the amount of heat needed to raise the temperature of one gram of a substance by 1°C, is often used instead. The symbol for specific heat is s. The specific heat of water is 4.18 J/g · °C.
Specific heat worksheet with answers. A 15 75 g piece of iron sorbs 1086 75 joules of heat energy and its temperature changes from 25 0 1750c. Heat is not the same as temperature yet they are related. Use q m δt cp to solve the following problems. Show all work and proper units. Perform calculations usin 1. Gold has a specific heat of 0 129 j gx0c. Specific heat worksheet with answers pdf. 4 A copper cylinder has a mass of 768 g and a specific heat of 0092 calgC. Specific heat capacity worksheet key specific heat capacity. How much heat energy joules must be removed to cause this cooling. She raises the temperature from 50 C to the same final temperature as problem 5. Muscle fatigue refers to the decline in muscle force generated over sustained periods of activity or due to pathological issues. Muscle fatigue has a number of possible causes including impaired blood flow, ion imbalance within the muscle, nervous fatigue, loss of desire to continue, and most importantly, the accumulation of lactic acid in the muscle. Sep 27, 2021 · Specific gravity is an important tool in the jewelry business. Let's imagine that Julie the Jeweler wants to make a piece with a gold ring. She buys the gold online and wants to know for sure that ...
temperature, determined to be 27.8 °C. Assuming no heat lost to the environment, calculate the specific heat of the metal. (Hint: First calculate the heat absorbed by the water then use this value for “Q” to determine the specific heat of the metal in a second calculation) Qwater= 1713.8 J Cmetal= 0.402 J/g oC 6. Specific Heat Worksheet Specific Heat DIRECTIONS: Use q = (m)(ΔT)(Cp) to solve the following problems. Show all work and units. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°C to 175°C. Calculate the specific heat capacity of iron. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°C to 175°C. Calculate the specific heat capacity of iron.5 pages Heat is not the same as temperature yet they are related. Use q m δt cp to solve the following problems. Pin On For Teacher . For q m oc a t. Specific heat worksheet answers. 2 what mass of water can be heated from 25 0 c to 50 0 c by the addition of 2825 j.Worksheet calculations involving specific heat 1.
Name Key. Specific Heat Worksheet. Cp = q/. mAT, where q = heat energy, m = mass, and T = temperature. 1. A 15.75-9 piece of iron absorbs 1086.75 joules of ...1 page
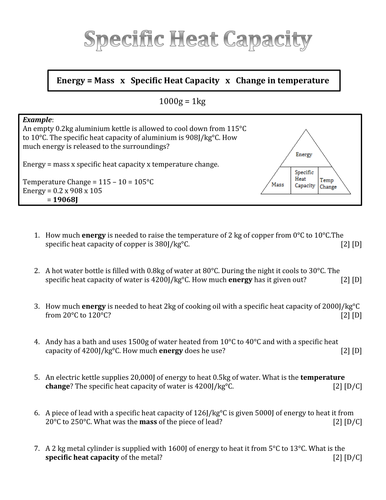
Specific Heat and Heat Capacity Worksheet DIRECTIONS: Use q = (m)(Cp))(ΔT) to solve the following problems. Show all work and units. Ex: How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C, if the specific heat of aluminum is 0.90 J/g°C? 1.
Specific Heat Worksheet Specific Heat Worksheet Name (in ink): C = q/m∆T, where q = heat energy, m = mass, and T = temperature Remember, ∆T = (Tfinal - Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1.
Each module of the series covers a different topic and is further broken down into sub-topics. A "MOP experience" will provide a learner with challenging questions, feedback, and question-specific help in the context of a game-like environment. It is available for phones, tablets, Chromebooks, and Macintosh computers.
The specific heat of aluminum is 0 901 j g oc. Whatever your company planning objectives cash flow is still the resource in the organization and managing money is the business purpose. When it burns a candle heats 45 grams of water from 21 to 28 c. Specific heat problems worksheet answers along with specific heat worksheet answers. What is the ...
File previews. docx, 18.76 KB. docx, 19.93 KB. xlsx, 17.87 KB. Two page worksheet using Specific Heat Capacity. Questions start easy then become gradually harder. Answers included on separate sheet. Also includes a spreadsheet to show how the calculations have been done.
Name:_____ Per:_____ Worksheet- Introduction to Specific Heat Capacities Heating substances in the sun: The following table shows the temperature after 10.0 g of 4 different substances have been in direct sunlight for up to 60 minutes.
Specific heat worksheet with answers. Show all work and proper units. Perform calculations usin 1. Worksheet calculations involving specific heat 1. A 15 75 g piece of iron sorbs 1086 75 joules of. Worksheet calculations involving specific heat. C q mat where q heat energy m mass and t temperature remember at tfinal tinitial. Use q m δt cp to ...
Specific heat capacity worksheet with answers. A piece of copper with a mass of 218 g has heat capacity of 83 9 j c what is the specific heat capacity of copper. View answer the mass of a metal that appears to be gold is 4 30 g. Identify each variables by name the units associated with it.
If the specific heat of copper is 390 J/g 0C, what is the change of the copper's temperature? Page 4. Answers. Q = mc∆T, where Q = heat energy, ...5 pages
Worksheet- Calculations involving Specific Heat 1. For q= m oc A T: identify each variables by name & the units associated with it. 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other. (-m.c.AT) a. Perform calculations usin 1. Gold has a specific heat of 0.129 J/(gx0C). How
Specific Heat Worksheet Name (in ink): C = q/mAT, where q = heat energy, m = mass, and T = temperature Remember, AT = (Tfinal — Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat energy, and its temperature changes from 25 0 1750C.
Worksheet- Calculations involving Specific Heat 1. For q= m c Δ T : identify each variables by name & the units associated with it. q = amount of heat (J) m = mass (grams) c = specific heat (J/g°C) ΔT = change in temperature (°C) 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other.
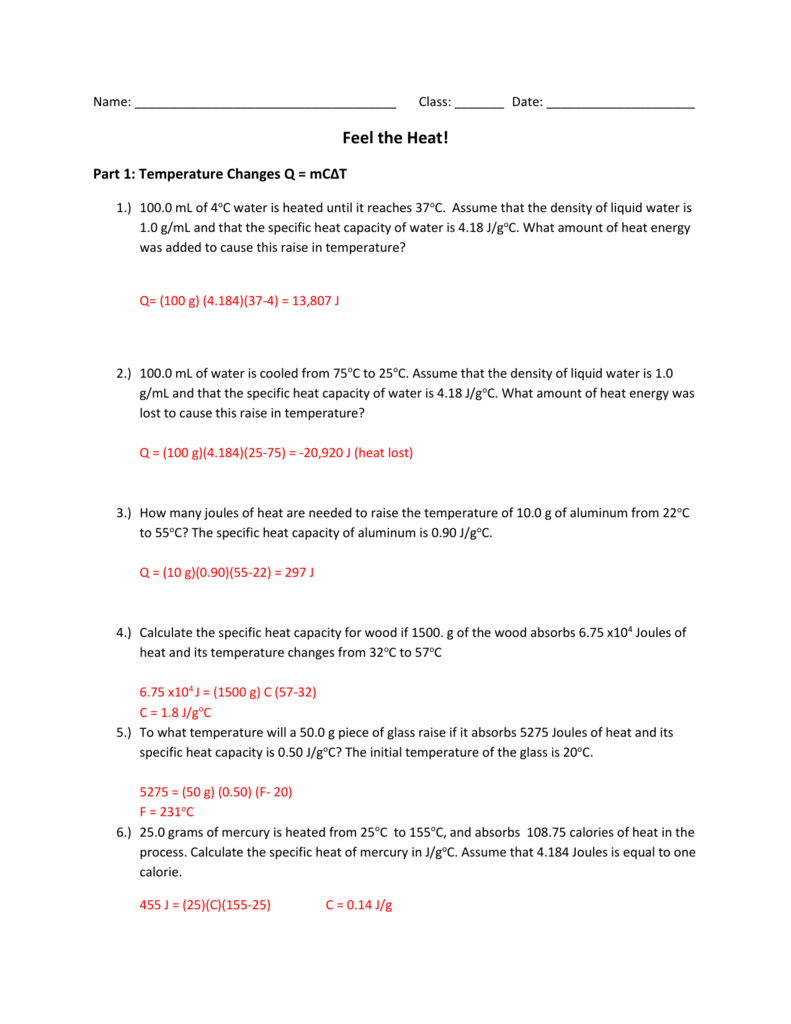
Answers to Worksheet 17 Calculating Heat The specific heat capacity c of a substance is the amount of heat required to raise the temperature of 1 gram of a substance by 1 K. Calculate the specific heat capacity of a piece of wood if 15000 g of the wood absorbs 67500 joules of heat and its temperature changes from 32c to 57c.
Chemistry Specific Heat Worksheet Answers Whether your kids are being educated in your home by you or in a classroom setup, you can provide these activities as research. If you plan to utilize these in a classroom, have students create their names, instructor names, section numbers, as well as the date before starting the task at hand.
Specific Heat and Heat Capacity Worksheet 1 The temperature of 335 g of water changed from 24.5oC to 26.4oC.How much heat did this sample absorb? c for water = 4.18 J/goC (ans. 2.66 kJ) 2.
Answers are provided at the end of the worksheet without units. O nb 25 a 75 0 g sample of a metal at is heated by adding 450 j of heat. Chemistry worksheet name specific heat capacity block. 4 18 j g 0 c 1 00 j g 0 c 0 80 j g 0 c. Calculate the energy required to heat a beaker of water at 18 c to boiling. This is an utterly simple.
A 1575 g piece of iron sorbs 108675 joules of heat energy and its temperature changes from 25 0 1750c. The mass of the water is 70 0 g. When the chemical reactions reach a specific temperature they are said to be complete. 100 0 ml of 4 0 c water is heated until its temperature is 37 c. Specific heat chem worksheet 16 1 answer key.















![Specific Heat Capacity - Worksheet (key) [d4p7my5q264p]](https://idoc.pub/img/crop/300x300/d4p7my5q264p.jpg)













0 Response to "42 specific heat worksheet answers"
Post a Comment